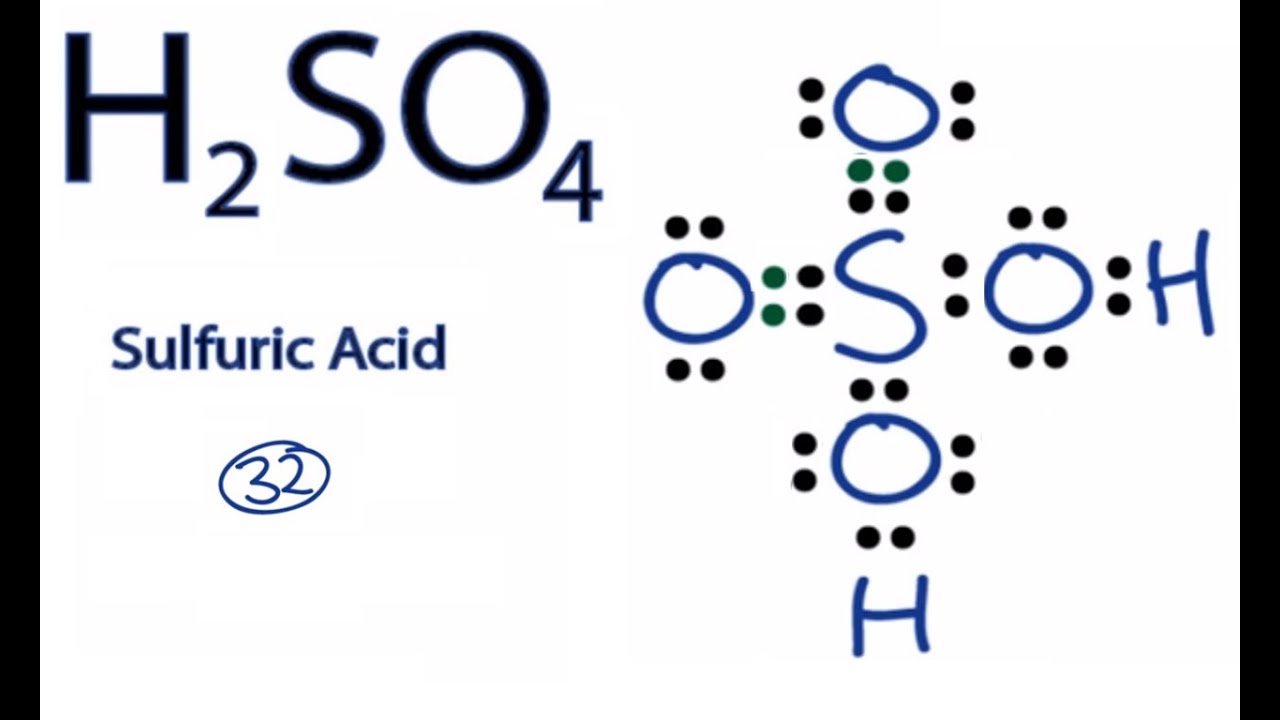
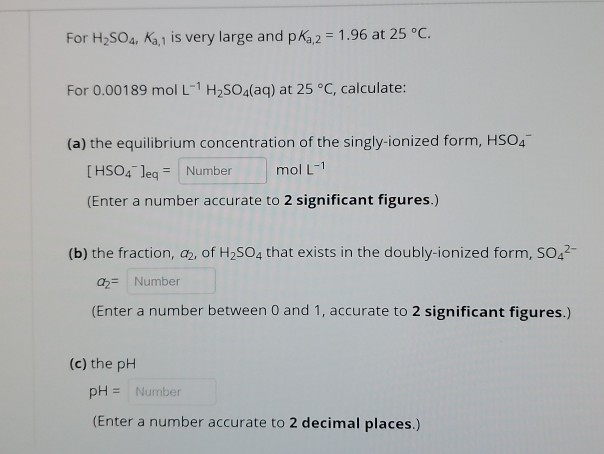
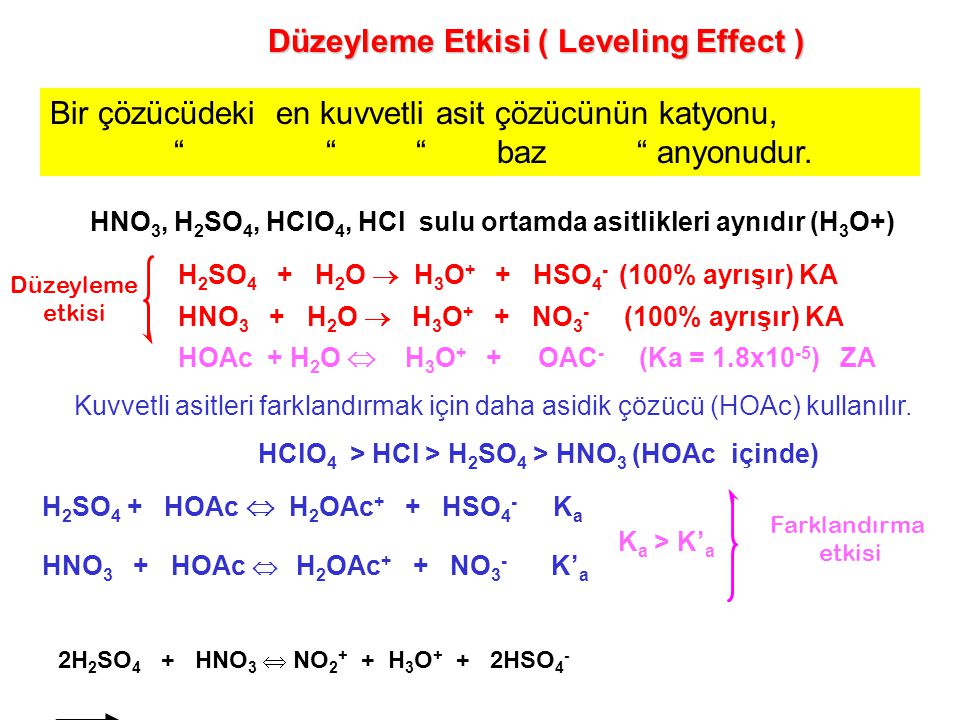
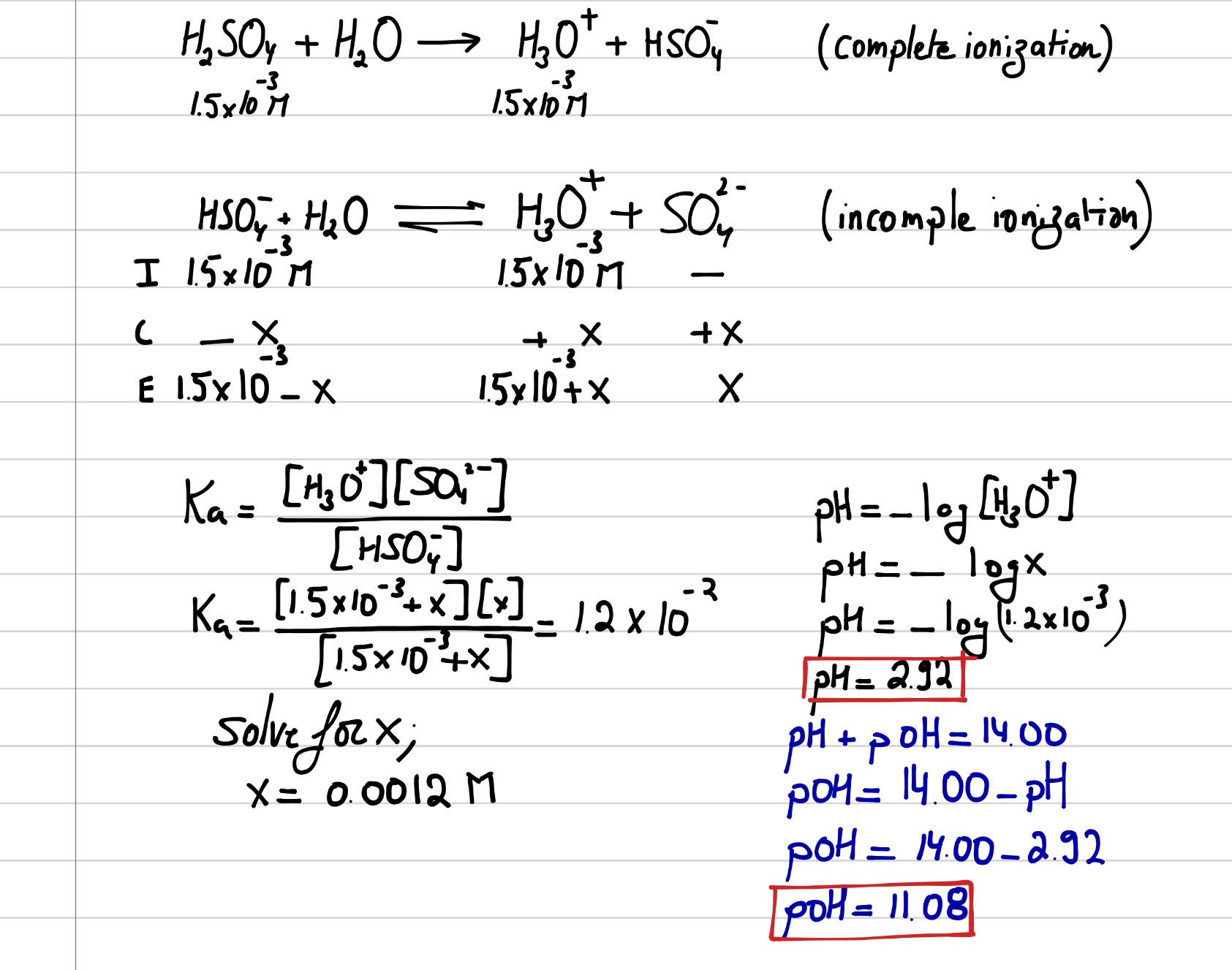SOLVED: PH of Aqueous Sulfuric Acid Calculate the pH of a 3.12x10^-3 M solution of H2SO4: (Ka = 0.0120 for HSO4)

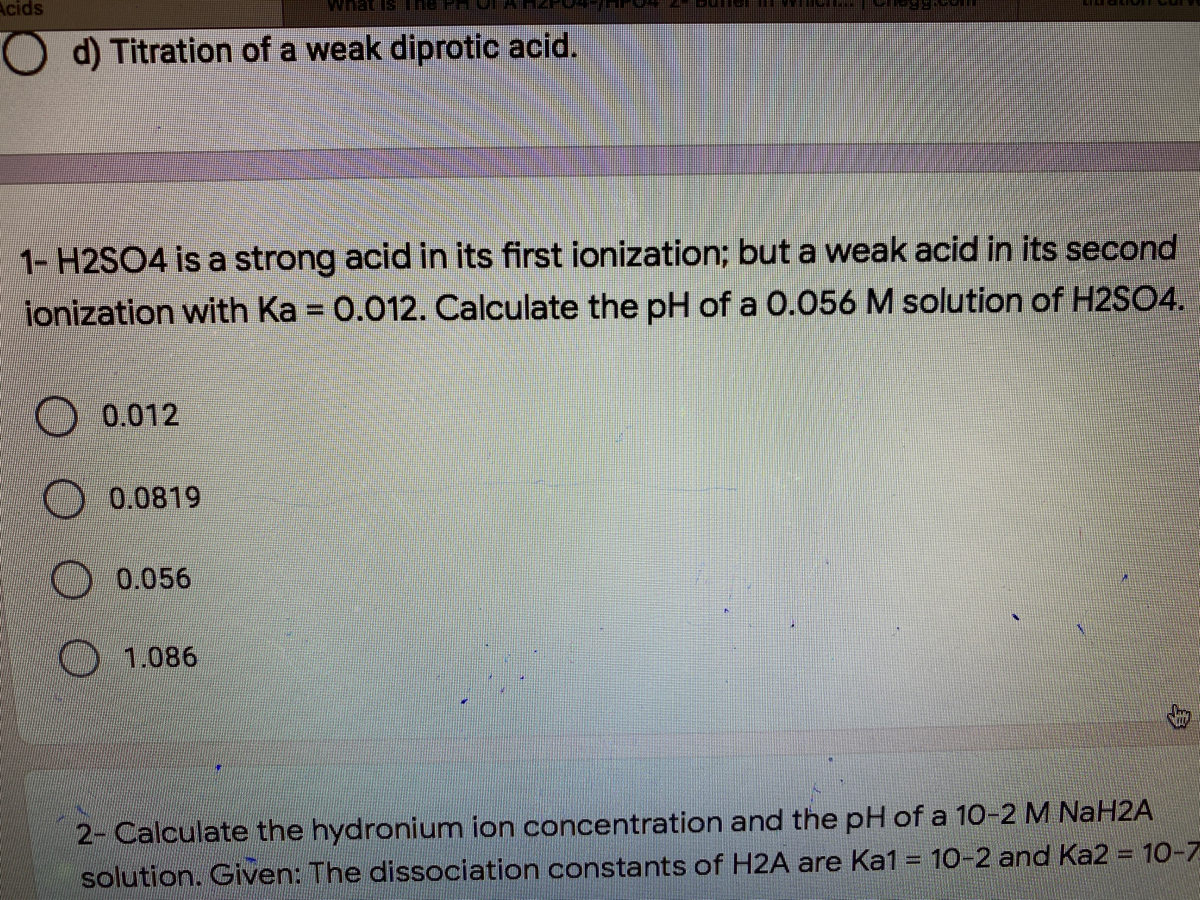
sir iss 3 question mei h2so4 ka Ph 0 7 kaise aagya kyuki h2so4 to diprotic hai isme two deprotanations - Chemistry - - 14691351 | Meritnation.com
How much H2SO4 (in gram) must be added in 500ml of 0.1M CH3COOH (Ka=10 5) to change its degree of dissociation by 200 times
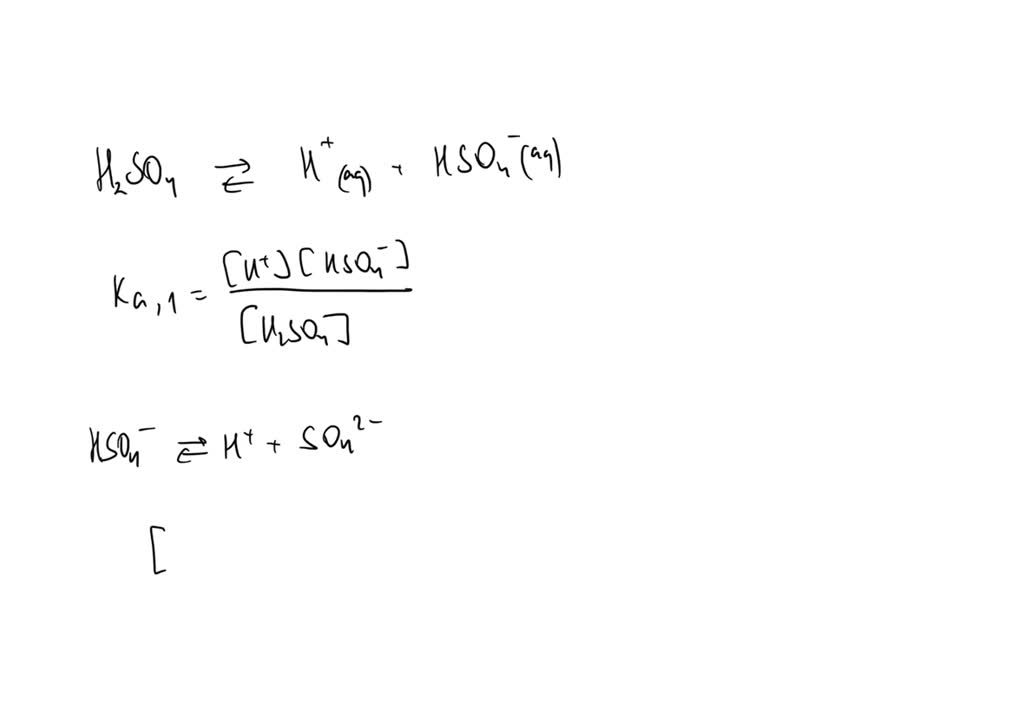
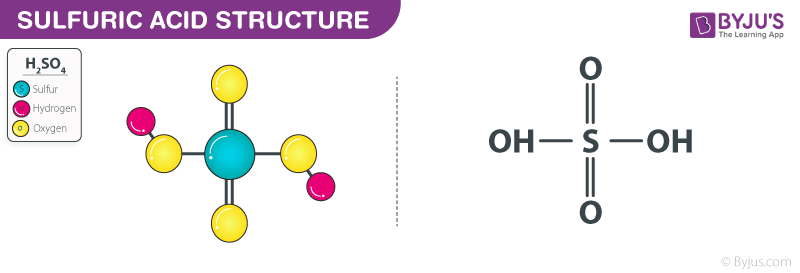
SOLVED: Write the acid dissociation equilibrium for H2SO4 in water and write the Ka expression. Is this a strong or weak acid? Are products, reactants, or both favored at equilibrium? Answer: This

Poliprotik Asitler Bünyesinde birden fazla iyonlaşabilen hidrojen içeren asitlerdir. Örneğin H2SO4,H3PO4 ve H2CO3 gibi … H3A şeklindeki bir poliprotik. - ppt indir